 Pic showing an officer explaining the registration of health product to a stall owner / retailer
Pic showing an officer explaining the registration of health product to a stall owner / retailer Pic showing the use of consumer education tools (phamplet and postcard) in the education campaign
Pic showing the use of consumer education tools (phamplet and postcard) in the education campaignFor all of Us who care about our health, the health of our loved ones, the use of medicine, use of healthcare product and general knowledge about enforcement and regulatory control of pharmaceuticals
 Pic showing an officer explaining the registration of health product to a stall owner / retailer
Pic showing an officer explaining the registration of health product to a stall owner / retailer Pic showing the use of consumer education tools (phamplet and postcard) in the education campaign
Pic showing the use of consumer education tools (phamplet and postcard) in the education campaign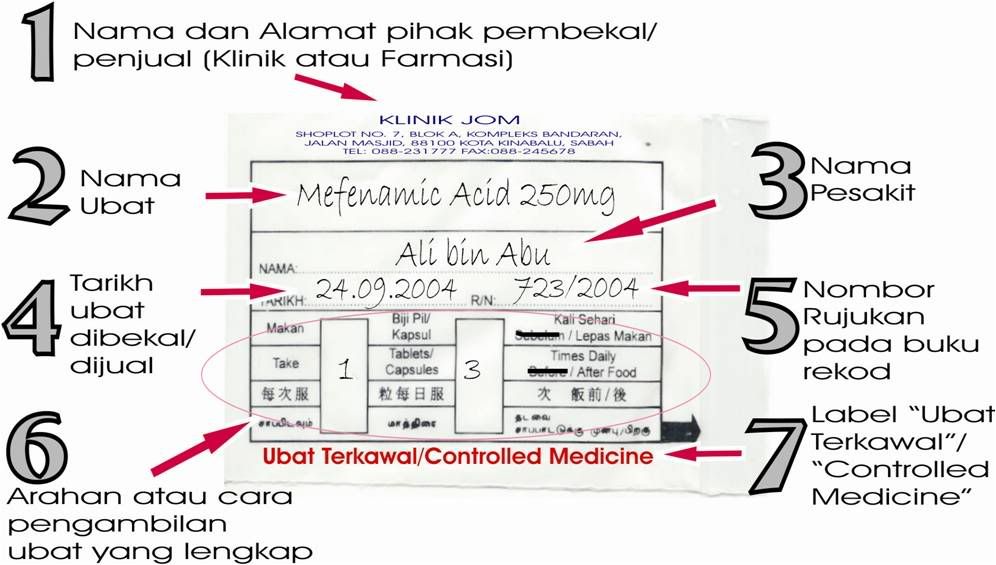
(Pic above shows the proper labelling of dispensed medicine)
Consumer Responsibility
The government always advocates Malaysians to be smart consumers. One small step to achieve this aspiration is to read the medicine label and to know the medicine supplied to you. It’s not a surprising fact that certain members of the public neglects to read the label of their medicine. Whether this medicine is obtained from hospital, clinic, or retail pharmacy, many of us don’t take the time to read or to ask about the medicine we received. Please find the time at the end of this article to check your medicine cabinet and look at the medicine label that many of us are currently taking, it’s not surprising to find dispensed medicine labelled with the words ‘Sakit Perut’, headache, ‘muntah-muntah’, ‘cuci darah’, and many more. Some might find this amusing and strange but the bottom line it’s a sad reality. The paradigm shift in medical care is to encourage the patient to play an active role in treatment of their illnesses. The patient can play this role by taking the initiative to ensure that their medicine is clearly and completely labeled. Request the doctor or pharmacist who supplies the medicine to label the medicines properly. All patients whether adults or children have the right to expect the medicine supplied to them are appropriate, safe and effective. By fulfilling this basic consumer responsibility it is hoped that consumer will be familiar with the medicine that they are taking and take more responsibility in their treatment.
Supplier Responsibility
An example of professional duty in the medical field is to inform matters related to any diseases or medicine involving such patients in accordance with the standard clinical guidelines that focuses on the patient (patient centered). All doctors, dentists or pharmacist must ensure that the label of dispensed medicine conforms to the law. In any government medical institutions, labeling of medicine with generic or chemical name has become a norm. Such practices should be implemented in all private hospitals, clinics and retail pharmacies for the benefit of the patients. For example the common painkiller medicine containing the chemical Mefenamic Acid has been registered undert the names like Ponstan, Beafemic, Dyfenalic, Fenagesic, Hamitan, Mefemic etc. The practice of labelling using chemical name is important because it allows the consumer to know the active ingredients in each medicine and help to avoid any toxicity that might arise due to the administration of different medicines with the same active ingredients. .
Advantage of Proper Labeling
The advantages of having a proper label of medicine are as follows;
1.The patient can obtain further information about that particular medicine from reliable information sources or by referring the medicine by name to doctors and pharmacists
2. Improve patients compliance to maximize the treatment effectiveness
3. Patients able tell the name of medicine they are taking to doctors or pharmacist to avoid duplication of therapy especially for those seeking second opinion from different healthcare provider for a particular illness.
4. If adverse effects or allergic reaction occur, it will be easy for patient to recognize the medicine that causes it and to report the matter to their medical provider.
5. The patient will be able to stop taking medicine which has an active ingredient which is banned
6. Easy to understand the direction, frequency and duration of treatment for that particular medicine
7. Know the medicine proper and safe storage
8. Avoid cases of medicine mistakenly consume by individuals living in the same house.
In conclusion, by taking the initiative to ensure your medicines are labelled properly, all of us choose to become smart consumers and take full charge in the treatment of our illness.
© Sabah State Pharmacy Enforcement Branch

 Photo showing products controlled by Drug Control Authority (DCA), Malaysia
Photo showing products controlled by Drug Control Authority (DCA), Malaysia More than half of the world population surfing this site is wondering what this sticker is for. It is being used by the authorities here in Malaysia to control health product in the Malaysian market. It serve a multi function role to tell the public that the product is registered. It also serve the industry to differentiate their product from wannabe imitations thus safeguarding their brand and income. It is also a useful tool for enforcement to facilitate their duties.
More than half of the world population surfing this site is wondering what this sticker is for. It is being used by the authorities here in Malaysia to control health product in the Malaysian market. It serve a multi function role to tell the public that the product is registered. It also serve the industry to differentiate their product from wannabe imitations thus safeguarding their brand and income. It is also a useful tool for enforcement to facilitate their duties.